PULBLICATIONS
Title: ####
Ryo Minamikawa, Rei Ishihara, Kohei Hatakeyama, Tatsuki Morimoto, and Satoshi Ueno,*
Submitted, 2025, ## (##), ####-####.
DOI: #####/####
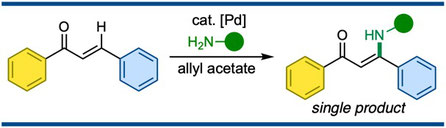
Cooperative Ruthenium/Amine Catalysis of the Cross-Coupling Reaction of Ketones as Alkenyl Electrophiles
Kohei Hatakeyama, Tatsuki Kawahara, Yuya Kogure, Tatsuki Morimoto, and Satoshi Ueno,*
J. Org. Chem. 2025, 90(12), 4409–4420.

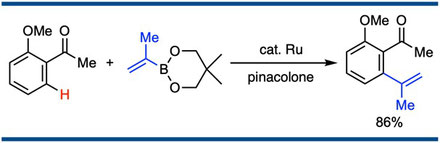
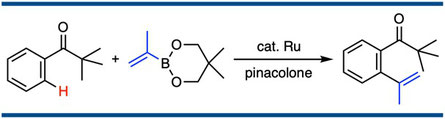
Ruthenium-Catalysed Cross-Coupling Reaction of Ketones with Transformable Directing Groups as Alkenyl Electrophiles
Yuya Kogure, Kohei Hatakeyama, Kai Tsuchiya, Yuta Kunii, and Satoshi Ueno,*
Chem. Commun. 2023, 59(83), 12463–12466.
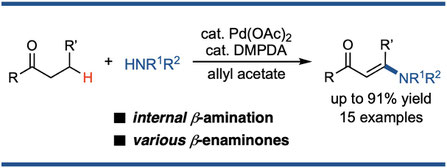
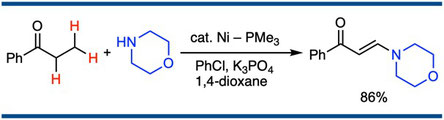
Synthesis of β-Enaminones via Palladium-Catalyzed Dehydrogeantive β-Amination of Saturated Ketones
Satoshi Ueno,* Shohei Yasuoka, Ryo Minamikawa, and Ryoichi Kuwano,*
Chem. Lett. 2023, 52(10), 783–787.
DOI: 10.1246/cl.230296 (Open Access)
(Selected as one of the Daily Top Accessed Articles in Chem. Lett., Oct. 2023)
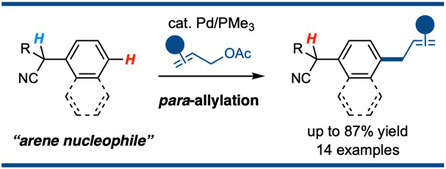
Palladium-Catalyzed para-Selective Allylation of 1-(Cyanomethyl)arenes with Allyl Acetates
Rina Muto, Kenji Nagata, Yoshiki Nakazumi, Kaho Nakamura, and Satoshi Ueno,*
Org. Lett. 2023, 25(12), 2108–2112.

One-Pot Synthesis of Substituted Pyridines from Alkyl Ketones and Enamines by the Nickel-Catalyzed Dehydrogenation of Alkyl Ketones
Satoshi Ueno,* Ryohei Maeda, Yuya Kogure, and Ryoichi Kuwano,*
Chem. Lett. 2023, 52(3), 148–151.
DOI: 10.1246/cl.220546 (Open Access)
(Selected as one of the Daily Top Accessed Articles in Chem. Lett., Mar. 2023)
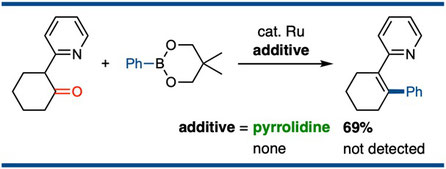
Ruthenium-Catalyzed Cross-Coupling of Ketones as an Alkenyl Electrophile with Organoborons via Cleavage of Alkenyl C–N Bonds of in Situ Generated Enamines
Yuya Kogure and Satoshi Ueno,*
Org. Lett. 2022, 24(50), 9233–9237.
DOI: 10.1021/acs.orglett.2c03765
(Selected as one of the Monthly Top 20 Most Read Articles in Org. Lett., Jan. 2023)

Diamine-Promoted Deacylation of 2-Alkyl-1,3-Diketones for the Facile Synthesis of Ketones
Rei Ishihara, Kota Okamura, Yuki Yoshimura, and Satoshi Ueno,*
ChemistrySelect, 2022, 7(37), e202202717.

One-Pot Synthesis of Multiarylated Benzophenones via [3 + 2 + 1] Benzannulation of Ketones, Alkynes, and α,β-Unsaturated Carbonyls
Shoko Nagahata, Seiya Takei, and Satoshi Ueno,*
J. Org. Chem. 2022, 87(15), 10377–10384.

Palladium-Catalyzed Dehydrogenative [3+3] Aromatization of Propyl Ketones and Allyl Carbonates
Kenta Koike and Satoshi Ueno,*
Chem. Lett. 2022, 51(4), 489–492.
DOI:10.1246/cl.220032 (Open Access)
(Selected as one of the Daily Top Accessed Articles in Chem. Lett., Apr. 2022)
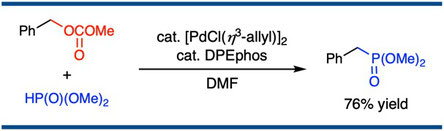
Palladium-catalyzed Benzylic Substitution of Benzyl Carbonates with Phosphorus Nucleophiles
Yusuke Makida, Kazumi Usui, Satoshi Ueno, and Ryoichi Kuwano,*
Chem. Lett. 2017, 46(12), 1814–1817.
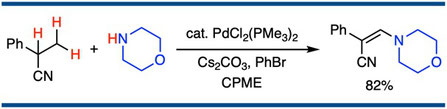
β-Amination of Saturated Nitriles through Palladium-Catalyzed Dehydrogenation/1,4-Addition/Re-dehydrogenation
Satoshi Ueno,* Ryohei Maeda, Shohei Yasuoka, and Ryoichi Kuwano,*
Chem. Lett. 2013, 42(1), 40–42.
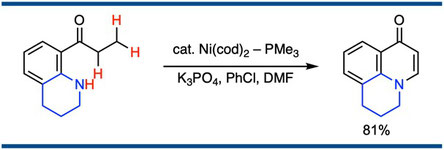
Synthesis of 4-Quinolones through Nickel-Catalyzed Intramolecular Amination on the β-Carbon of o-(N-Alkylamino)propiophenones
Satoshi Ueno,* Ryosuke Shimizu, Ryohei Maeda, Ryoichi Kuwano,*
Synlett 2012, 23(11), 1639–1642.
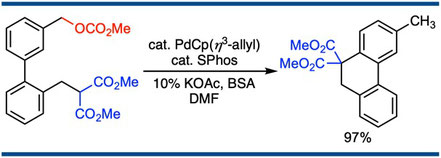
Intramolecular SN’-Type Aromatic Substitution of Benzylic Carbonates at their Para-Position
Satoshi Ueno, Sadakazu Komiya, Takeshi Tanaka, and Ryoichi Kuwano,*
Org. Lett. 2012, 14(1), 338–341.
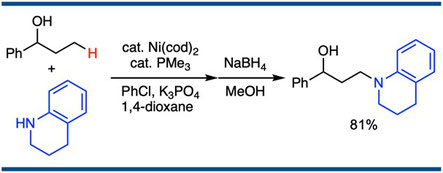
Transformation of α-Substituted Propanols to γ-Amino Alcohols through Nickel-Catalyzed Amination on the Terminal γ-Carbon of Propanols
Satoshi Ueno,* Kazumi Usui, and Ryoichi Kuwano,*
Synlett 2011, (9), 1303–1307.

Palladium-Catalyzed [4+2] Cycloaddition of o-(Silylmethyl)benzyl Esters with Ketones: An Equivalent to Oxo-Diels–Alder Reaction of o-Xylylenes
Satoshi Ueno, Masakazu Ohtsubo, and Ryoichi Kuwano,*
Org. Lett. 2010, 12(19), 4332–4334.
Nickel-Catalyzed Formation of a Carbon–Nitrogen Bond at the β-Position of Saturated Ketones
Satoshi Ueno,* Ryosuke Shimizu, and Ryoichi Kuwano,*
Angew. Chem. Int. Ed. 2009, 48(25), 4543–4545.
(Highlighted in Speciality Chemicals Magazine: February 2010 Chemistry Corner)

[4+2] Cycloaddition of o-Xylylenes with Imines Using Palladium Catalyst
Satoshi Ueno, Masakazu Ohtsubo, and Ryoichi Kuwano,*
J. Am. Chem. Soc. 2009, 131(36), 12904–12905.
Unique Effect of Coordination of an Alkene Moiety in Products on Ruthenium-Catalyzed Chemoselective C–H Alkenylation
Satoshi Ueno, Takuya Kochi, Naoto Chatani, and Fumitoshi Kakiuchi,*
Org. Lett. 2009, 11(4), 855–858.

Direct, Iridium-Catalyzed Enantioselective and Regioselective Allylic Etherification with Aliphatic Alcohols
Satoshi Ueno and John F. Hartwig,*
Angew. Chem. Int. Ed. 2008, 47(10), 1928–1931.

Enantioselective Iridium-Catalyzed Allylic Amination of Ammonia and Convenient Ammonia Surrogates
Mark J. Pouy, Andreas Leitner, Daniel J. Weix, Satoshi Ueno, and John F. Hartwig,*
Org. Lett. 2007, 9(20), 3949–3952.

Ruthenium-Catalyzed Carbon–Carbon Bond Formation via the Cleavage of an Unreactive Aryl Carbon–Nitrogen Bond in Aniline Derivatives with Organoboronates
Satoshi Ueno, Naoto Chatani, and Fumitoshi Kakiuchi,*
J. Am. Chem. Soc. 2007, 129(19), 6098–6099.
Regioselective Alkenylation of Aromatic Ketones with Alkenylboronates Using a RuH2(CO)(PPh3)3-Catalyst via Carbon–Hydrogen Bond Cleavage
Satoshi Ueno, Naoto Chatani, and Fumitoshi Kakiuchi,*
J. Org. Chem. 2007, 72(9), 3600–3602.
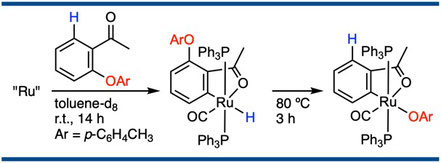
Direct Observation of the Oxidative Addition of the Aryl Carbon–Oxygen Bond to a Ruthenium Complex and Consideration of the Relative Reactivity between Aryl Carbon–Oxygen and Aryl Carbon–Hydrogen Bonds
Satoshi Ueno, Eiichiro Mizushima, Naoto Chatani, and Fumitoshi Kakiuchi,*
J. Am. Chem. Soc. 2006, 128(51), 16516–16517.

Ruthenium-Catalyzed Functionalization of Aryl Carbon–Oxygen Bonds in Aromatic Ethers with Organoboron Compounds
Fumitoshi Kakiuchi,* Mayumi Usui, Satoshi Ueno, Naoto Chatani, and Shinji Murai,
J. Am. Chem. Soc. 2004, 126(9), 2706–2707.
東京工科大学工学部応用化学科
東京工科大学大学院工学研究科
有機合成化学(上野)研究室
〒192-0982 東京都八王子市片倉町1404-1
東京工科大学片柳研究所棟KW402
Department of Applied Chemistry, School of Engineering
Graduate School of Engineering
Tokyo University of Technology
Synthetic Organic Chemistry Laboratory (Ueno Group)
1404-1 Katakura, Hachioji, 192-0982, Tokyo, Japan
Katayanagi Advanced Research Institute KW402
